
Smartphone-powered dongle tests for HIV in 15 minutes
News: Columbia Engineering researchers have developed a smartphone accessory that can simultaneously detect HIV and syphilis within minutes.
A team led by professor Samuel K Sia, associate professor of biomedical engineering at Columbia University's engineering school, designed the low-cost smartphone add-on, which has already been tested by healthcare workers in Rwanda.
The device emulates an enzyme-linked immunosorbent assay, or ELISA – a blood test usually carried out in a lab – using the phone's audio jack as a power source.
An app prompts the user to enter a patient ID and then displays instructions to guide them through the process.
Users first disinfect a finger and obtain a finger-prick of blood for testing. This is placed into a plastic collector and then inserted into a special chip.
A disposable cassette containing the chip with the blood sample is then inserted into the dongle. Pressing a bulb activates the testing process, and 15 minutes later the results are displayed on-screen.
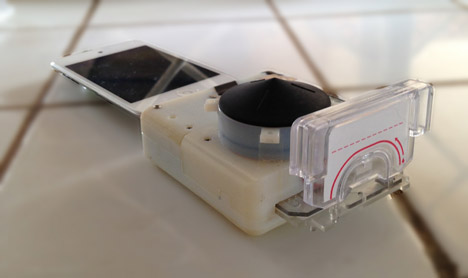
The disposable cassettes contain reagents, a substance which normally contains an antibody that will stick to a protein in a blood sample – in this case an HIV or syphillis viral protein.
When mixed with the blood, the reagent creates a chemical reaction that will make the substance change colour if the viral proteins are present. An enzyme is included to help speed up this process. Any change is then detected by shining light on the substance and measuring how much is absorbed. The results of this indicate the presence of the viral protein.
In standard equipment, this mixing is achieved used an electrically-powered pump. In the dongle, this has been replaced with a "one-push vacuum" – a soft plastic button that can be compressed and released to create negative pressure in the testing chamber.
According to the team, the dongle, which can also be connected to a computer, matches the quality of a full laboratory test.
"It performs a triplexed immunoassay not currently available in a single test format: HIV antibody, treponemal-specific antibody for syphilis, and non-treponemal antibody for active syphilis infection," explained a statement from Columbia Engineering.
"The team developed the dongle to be small and light enough to fit into one hand, and to run assays on disposable plastic cassettes with pre-loaded reagents, where disease-specific zones provided an objective read-out."
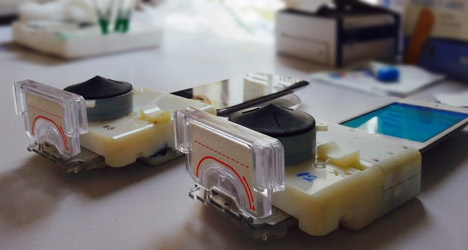
According to Sia, the dongle could have a manufacturing cost of $34, compared to the $18,450 cost for equipment typically used for these tests.
Although many areas do not have reliable access to electricity, there are now more than 7 billion mobile devices in the world. This led the team to focus on designing a device that could be powered by just a smartphone.
The dongle plugs into the audiojack rather than the phone's power dock; connecting cables for audio equipment have been standardised around the world, so this allows the device to be virtually universal.
"Our dongle presents new capabilities for a broad range of users, from health care providers to consumers," explained professor Samuel K Sia. "By increasing detection of syphilis infections, we might be able to reduce deaths by 10-fold."
"And for large-scale screening where the dongle's high sensitivity with few false negatives is critical, we might be able to scale up HIV testing at the community level with immediate antiretroviral therapy that could nearly stop HIV transmissions and approach elimination of this devastating disease," he said.
During field testing in Rwanda, care workers were given just 30 minutes of training. According to the researchers, patients responded well and said they would recommend the device to others.
Sia collaborated with researchers from Columbia’s Mailman School of Public Health; the Institute of HIV Disease Prevention and Control, Rwanda Biomedical Center; Department of Pathology and Cell Biology, Columbia University Medical Center; Centers for Disease Control and Prevention—Laboratory Reference and Research Branch, Atlanta; and OPKO Diagnostics.